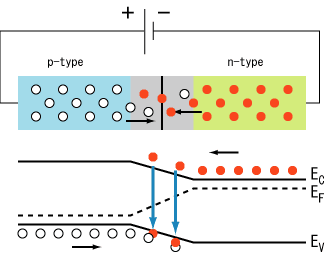
WORKING OF AN LED
Free electrons moving across a diode can fall into empty holes from the P-type layer. This involves a drop from the conduction band to a lower orbital, so the electrons release energy in the form of photons. This happens in any diode, but you can only see the photons when the diode is composed of certain material. The atoms in a standard silicon diode, for example, are arranged in such a way that the electron drops a relatively short distance. As a result, the photon's frequency is so low that it is invisible to the human eye -- it is in the infrared portion of the light spectrum. This isn't necessarily a bad thing, of course: Infrared LEDs are ideal for remote controls, among other things.
Visible
light-emitting diodes (VLEDs), such as the ones that light up numbers
in a digital clock, are made of materials characterized by a wider
gap between the conduction band and the lower orbitals. The size of
the gap determines the frequency of the photon -- in other words, it
determines the color of the light. While LEDs are used in everything
from remote controls to the digital displays on electronics, visible
LEDs are growing in popularity and use thanks to their long lifetimes
and miniature size. Depending on the materials used in LEDs, they can
be built to shine in infrared, ultraviolet, and all the colors of the
visible spectrum in between.

Light
is a form of energy that can be released by an atom. It is made up of
many small particle-like packets that have energy and momentum but no
mass. These particles, called photons, are the most basic units of
light.
Photons
are released as a result of moving electrons. In an atom, electrons
move in orbitals around the nucleus. Electrons in different orbitals
have different amounts of energy. Generally speaking, electrons with
greater energy move in orbitals farther away from the nucleus.
For
an electron to jump from a lower orbital to a higher orbital,
something has to boost its energy level. Conversely, an electron
releases energy when it drops from a higher orbital to a lower one.
This energy is released in the form of a photon. A greater energy
drop releases a higher-energy photon, which is characterized by a
higher frequency.
Free electrons moving across a diode can fall into empty holes from the P-type layer. This involves a drop from the conduction band to a lower orbital, so the electrons release energy in the form of photons. This happens in any diode, but you can only see the photons when the diode is composed of certain material. The atoms in a standard silicon diode, for example, are arranged in such a way that the electron drops a relatively short distance. As a result, the photon's frequency is so low that it is invisible to the human eye -- it is in the infrared portion of the light spectrum. This isn't necessarily a bad thing, of course: Infrared LEDs are ideal for remote controls, among other things.

1 comments:
Surprising background experienced in everything sentence of this article. I made a decent attempt to get hint about how I could demonstrate substance of this blog. I must say, not much powerful but rather I surrendered every one of my weapons soon after understanding it.หลอด ไฟ แอ ล อี ดี
Post a Comment