Light emitting diodes, commonly called LEDs, are real unsung heroes in the electronics world. They do dozens of different jobs and are found in all kinds of devices. Among other things, they form numbers on digital clocks, transmit information from remote controls, light up watches and tell you when your appliances are turned on. Collected together, they can form images on a jumbo television screen or illuminate a traffic light.
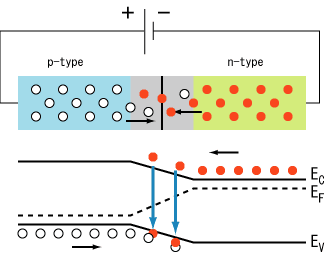
Basically, LEDs are just tiny light bulbs that fit easily into an electrical circuit. But unlike ordinary incandescent bulbs, they don't have a filament that will burn out, and they don't get especially hot. They are illuminated solely by the movement of electrons in a semiconductor material, and they last just as long as a standard transistor. The lifespan of an LED surpasses the short life of an incandescent bulb by thousands of hours. Tiny LEDs are already replacing the tubes that light up LCD HDTVs to make dramatically thinner televisions.
In this article, we'll examine the technology behind these ubiquitous blinkers, illuminating some cool principles of electricity and light in the process.
EARLY DISCOVERIES:
Electroluminescence as a phenomenon was discovered in 1907 by the British experimenter H. J. Round of Marconi Labs, using a crystal of silicon carbide and a cat's-whisker detector.Russian Oleg Vladimirovich Losev reported creation of the first LED in 1927. His research was distributed in Russian, German and British scientific journals, but no practical use was made of the discovery for several decades.Rubin Braunstein of the Radio Corporation of America reported on infrared emission from gallium arsenide (GaAs) and other semiconductor alloys in 1955.Braunstein observed infrared emission generated by simple diode structures using gallium antimonide (GaSb), GaAs, indium phosphide (InP), and silicon-germanium (SiGe) alloys at room temperature and at 77 kelvin.
In 1961, American experimenters Robert Biard and Gary Pittman working at Texas Instruments, found that GaAs emitted infrared radiation when electric current was applied and received the patent for the infrared LED.
The first practical visible-spectrum (red) LED was developed in 1962 by Nick Holonyak Jr., while working at General Electric Company. Holonyak is seen as the "father of the light-emitting diode".M. George Craford, a former graduate student of Holonyak, invented the first yellow LED and improved the brightness of red and red-orange LEDs by a factor of ten in 1972. In 1976, T.P. Pearsall created the first high-brightness, high efficiency LEDs for optical fiber telecommunications by inventing new semiconductor materials specifically adapted to optical fiber transmission wavelengths.
Until 1968, visible and infrared LEDs were extremely costly, on the order of US $200 per unit, and so had little practical use. The Monsanto Company was the first organization to mass-produce visible LEDs, using gallium arsenide phosphide in 1968 to produce red LEDs suitable for indicators. Hewlett Packard (HP) introduced LEDs in 1968, initially using GaAsP supplied by Monsanto. The technology proved to have major uses for alphanumeric displays and was integrated into HP's early handheld calculators. In the 1970s commercially successful LED devices at under five cents each were produced by Fairchild Optoelectronics. These devices employed compound semiconductor chips fabricated with the planar process invented by Dr. Jean Hoerni at Fairchild Semiconductor. The combination of planar processing for chip fabrication and innovative packaging methods enabled the team at Fairchild led by optoelectronics pioneer Thomas Brandt to achieve the needed cost reductions. These methods continue to be used by LED producers.
The first commercial LEDs were commonly used as replacements for incandescent and neon indicator lamps, and in seven- segment displays,first in expensive equipment such as laboratory and electronics test equipment, then later in such appliances as TVs, radios, telephones, calculators, and even watches (see list of signal uses). These red LEDs were bright enough only for use as indicators, as the light output was not enough to illuminate an area. Later, other colors grew widely available and also appeared in appliances and equipment. As LED materials technology grew more advanced, light output rose, while maintaining efficiency and reliability at acceptable levels.
▪ Visual signals where light goes more or less directly from the source to the human eye, to convey a message or meaning.
▪ Illumination where light is reflected from objects to give visual response of these objects.
▪ Measuring and interacting with processes involving no human vision.[100]
▪ Narrow band light sensors where LEDs operate in a reverse-bias mode and respond to incident light, instead of emitting light.